

Drawing on reaction rate 3 or dynamical 4 theories to compute the average jump rate, it has become customary to interpret the temperature dependence of D using the semi-empirical relation, \(D(T)=g\ f\ \nu \exp \left((\Delta \) denote positive vibrational frequencies at GS and TS, respectively.

In crystals, where sequences of diffusive events are usually dominated by a single-jump mechanism, D is proportional to the product of the average rate at which jumps are activated and the concentration of defects possibly mediating jumps. Entropy is a property while enthalpy is a type of energy Entropy is denoted as s while enthalpy as H. In this video you will come to understand what is enthalpy and change in enthalpy, also what is entropy in the system, also enthalpy in constant pressure pr. We then show that, on average, variations of atomic interactions along diffusion reaction paths simultaneously soften low frequency phonons and stiffen high frequency ones because relative frequency variations are larger in the lower region of the spectrum, softening generally prevails over stiffening and entropy ubiquitously increases with energy.Ītomic diffusion in solids proceeds via sequences of thermally activated atomic jumps, which, in the presence of chemical inhomogeneities, induce a flux of atoms that can be described at the mesoscopic level by the diffusion coefficient D 1, 2. The S.I unit of entropy is JK-1 while that of enthalpy is Jmol-1 Entropy is measured by the difference between the heat change of the chemical process and the temperature while enthalpy represents heat change at standard conditions.
Enthalpy vs entropy free#
Enthalpy in Intensive Units Specific Enthalpy. Enthalpy, Entropy & Gibbs Free Energy Enthalpy is the amount of heat energy transferred (heat absorbed or emitted) in a chemical process under. The word ‘enthalpy’ for this measure was coined by a 19 th-century famous physicist Heike Kamerlingh Onnes. The word enthalpy is derived from the Greek word ‘enthalpos,’ meaning ‘to put heat into’. the work done is: W pV 500 000 Pa x 1.34 m 3 670 kJ. It tells about the difference between the enthalpy of the products and the enthalpy of the reactants. Second law: In an isolated system, natural processes are spontaneous when they lead to an increase in disorder, or entropy. First law: Energy is conserved it can be neither created nor destroyed.
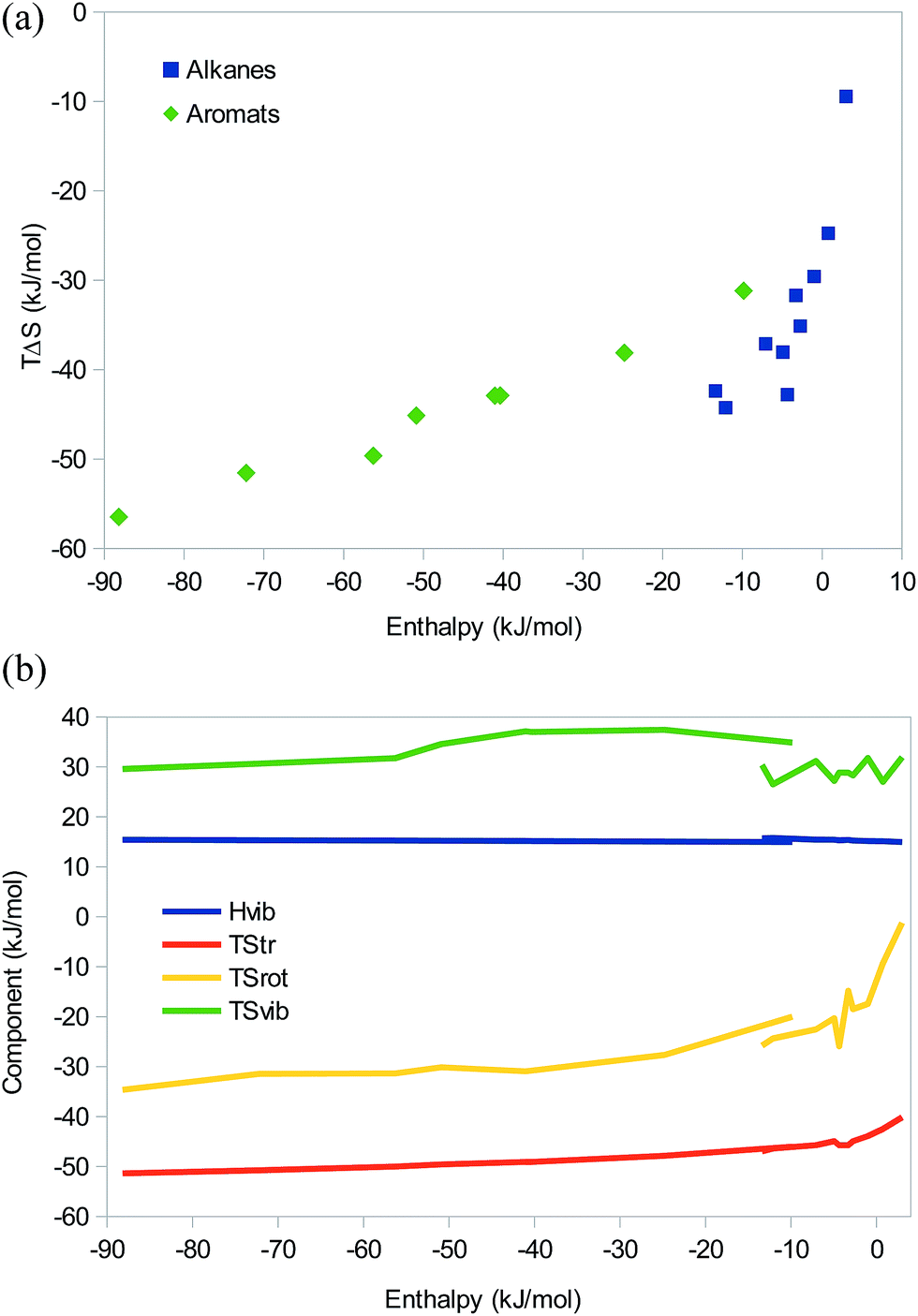
Here, we solve this decades-old problem by demonstrating that atomistically computed harmonic vibrational entropic contributions account for most of compensation effects in silicon and aluminum. in isentropic process, the enthalpy change equals the flow process work done on or by the system. Chemical thermodynamics is the portion of thermodynamics that pertains to chemical reactions. However, no physical model of entropy has ever been successfully tested against experimental compensation data. This so-called compensation effect has been argued to result from a universal positive linear relationship between entropic contributions and energy barriers to diffusion. There are two types of reactions related to enthalpy: Exothermic - where energy (in the form of heat) is released. This will cause further differences between their heat capacities.Experimental data accumulated over more than 120 years show not only that diffusion coefficients of impurities ordinarily obey the Arrhenius law in crystalline solids, but also that diffusion pre-exponential factors measured in a same solid increase exponentially with activation energies. Enthalpy is a measure of the amount of energy that exists in a system at constant pressure. Thus they will not always be of the same phase. In addition, hydrogen and deuterium have different freezing and boiling points. In each case the partition function $z$ has to be calculated then the entropy via $\displaystyle S=R\ln(z)+RT\left(\frac$ gives us the entropy by summing up these changes. It is defined as the sum of the enthalpy of a system and the product of. He termed it as available energy of a system that can be used to do work. Josiah Willard Gibbs developed Gibbs energy in the 1870s. The translational part is given by the Sakur-Tetrode eqn., the rotational part depends on the moment of inertia, and the vibrational part on the vibrational frequency. The relationship between enthalpy and entropy: The relationship between enthalpy and entropy can be seen to calculate the Gibbs free energy. Relation Between Enthalpy and Entropy Click Here for Sample Questions In the year 1870, Josiah Willard Gibbs found the Gibbs energy which uses systems enthalpy and products entropy along with the temperature of the system to calculate the total available energy of a system.


 0 kommentar(er)
0 kommentar(er)
